Latest Facts About Reverse OsmosisPosted by Daphne on April 20th, 2021 Need Reverse Osmosis Advice?Water filtration process Opposite osmosis (RO) is a water purification procedure that uses a partially absorptive membrane layer to separate ions, unwanted particles as well as bigger particles from alcohol consumption water. Backwards osmosis, an employed stress is used to get over osmotic pressure, a colligative home that is driven by chemical potential differences of the solvent, a thermodynamic criterion. Reverse osmosis can get rid of numerous sorts of dissolved and also suspended chemical species in addition to biological ones (primarily microorganisms) from water, and is utilized in both commercial processes as well as the manufacturing of drinkable water. The result is that the solute is preserved on the pressurized side of the membrane as well as the pure solvent is allowed to pass to the opposite side.
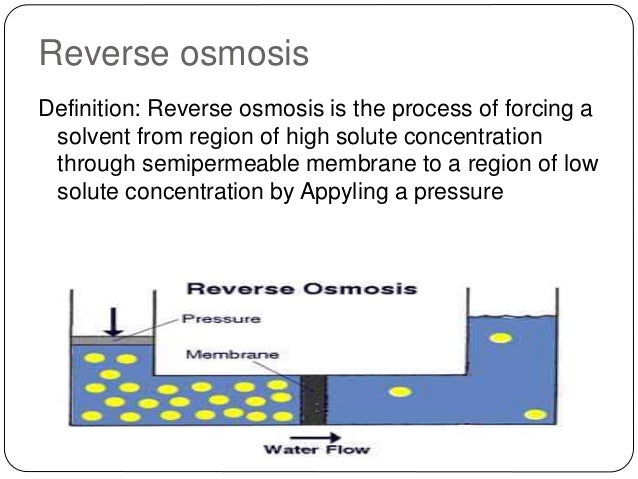
e., water, H2O) to pass easily. In the typical osmosis procedure, the solvent normally moves from a location of low solute focus (high water possibility), via a membrane, to a location of high solute concentration (reduced water capacity). The driving force for the motion of the solvent is the decrease in the Gibbs cost-free energy of the system when the distinction in solvent concentration on either side of a membrane is decreased, producing osmotic stress because of the solvent moving into the more focused remedy. Applying an external stress to turn around the natural circulation of pure solvent, therefore, is reverse osmosis. Reverse osmosis differs from filtering because the mechanism of fluid circulation is by osmosis across a membrane layer. The predominant removal mechanism in membrane filtration is stressing, or dimension exemption, where the pores are 0. 01 micrometers or larger, so the procedure can theoretically attain excellent performance despite criteria such as the remedy's stress and also focus. Reverse osmosis instead includes solvent diffusion across a membrane layer that is either nonporous or utilizes nanofiltration with pores 0. 001 micrometers in dimension. The predominant removal device is from distinctions in solubility or diffusivity, as well as the procedure is dependent on pressure, solute focus, as well as various other conditions. A procedure of osmosis via semipermeable membranes was initial observed in 1748 by Jean-Antoine Nollet. For the adhering to 200 years, osmosis was just a sensation observed busy. In 1950, the University of California at Los Angeles first investigated desalination of seawater using semipermeable membrane layers. Researchers from both College of California at Los Angeles as well as the College of Florida effectively generated fresh water from salt water in the mid-1950s, yet the flux was too low to be commercially feasible till the discovery at College of California at Los Angeles by Sidney Loeb and Srinivasa Sourirajan at the National Research Study Council of Canada, Ottawa, of techniques for making asymmetric membrane layers defined by a properly slim "skin" layer supported atop a very permeable and much thicker substrate region of the membrane layer. Information Around Reverse OsmosisCadotte's patent on this process was the subject of lawsuits and also has actually given that ended. Mostly all industrial reverse-osmosis membrane is now made by this method. By 2019, there were approximately 16,000 desalination plants operating worldwide, generating around 95 million m3/day of desalinated water for human usage. Around half of this capability was in the Middle East as well as North Africa area. Reverse osmosis production train, North Cape Coral Reverse Osmosis Plant, In 1977 Cape Reefs, Florida came to be the initial town in the USA to use the RO process on a big scale with a first operating ability of 11. By 1985, because of the fast growth in populace of Cape Coral reefs, the city had the biggest low-pressure reverse-osmosis plant worldwide, capable of generating 56. 8 million litres (15 million US gal) each day (MGD). Officially, reverse osmosis is the process of forcing a solvent from a region of high solute focus via a semipermeable membrane to an area of low-solute concentration by using a stress in unwanted of the osmotic stress. The biggest and crucial application of reverse osmosis is the separation of distilled water from seawater as well as brackish waters; seawater or briny water is pressurized versus one surface of the membrane, creating transportation of salt-depleted water across the membrane layer and introduction of drinkable drinking water from the low-pressure side.
In many cases, the membrane layer is developed to allow only water to go through this thick layer while preventing the flow of solutes (such as salt ions). This process needs that a high pressure be put in on the high-concentration side of the membrane layer, normally 217 bar (30250 psi) for fresh and also briny water, and also 4082 bar (6001200 psi) for salt water, which has around 27 bar (390 psi) all-natural osmotic stress that have to relapse. This process is best understood for its use in desalination (eliminating the salt and also other minerals from sea water to create fresh water), yet considering that the very early 1970s, it has actually additionally been made use of to cleanse fresh water for medical, industrial and residential applications. Such systems normally consist of a variety of steps: a sediment filter to catch fragments, consisting of corrosion and also calcium carbonate additionally, a second debris filter with smaller sized pores an triggered carbon filter to trap organic chemicals and also chlorine, which will attack and weaken specific kinds of thin-film composite membrane layer a reverse osmosis filter, which is a thin-film composite membrane additionally, an ultraviolet lamp for sterilizing any type of germs that may run away filtering by the reverse osmosis membrane layer optionally, a second carbon filter to catch those chemicals not removed by the reverse osmosis membrane layer In some systems, the carbon prefilter is omitted, and a cellulose triacetate membrane layer is used. Like it? Share it!More by this author |


 How to Choose the Right Reverse Osmosis
How to Choose the Right Reverse Osmosis Best Reverse Osmosis
Best Reverse Osmosis