According to recent industry analysis on the U.S. respiratory syncytial virus vaccines market by Persistence Market Research, a leading market research company, the industry is projected to be valued at US$ 320.3 Mn in 2022, and is set to expand at a CAGR of 18.2% over the next ten years. Demand for RSV vaccines in the U.S. is expected to increase manifold due to a large numbers of ongoing clinical trials and a strong product pipeline displaying promising results.
During the past 10 years, considerable progress has been made in RSV vaccine development. Key manufacturers of these vaccines are using monoclonal antibodies to cause passive immunization in infants. At present, there are around 17 new RSV vaccines under clinical development, which include live attenuated, vector-based, particle-based, and subunit vaccines.
Get Sample Copy of this Report @ https://www.persistencemarketresearch.com/samples/33045
Presently, key market players such as GSK, Janssen, Moderna, and Pfizer have global phase III trials underway for RSV vaccines, which reflects a great opportunity for industrial growth along with the launch of these products into the market in the future. Increasing funding from government bodies plays a vital role in driving demand for the market by supporting R&D activities in the country.
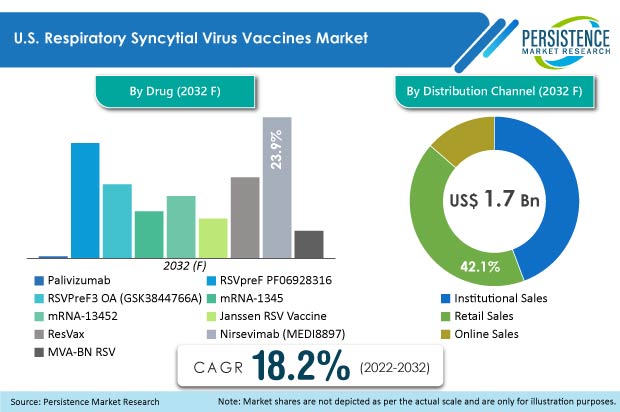
Thus, active participation of key players in clinical trials and financial help from the government will contribute in driving the respiratory syncytial virus vaccines market in the U.S. to reach a valuation of US$ 1.7 Bn by the end of 2032.
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/33045
Key Takeaways from Market Study
- By drug, Nirsevimab (MEDI8897) is projected to dominate the U.S. market with US$ 406.6 Mn valuation by 2032.
- Based on distribution channel, institutional sales are expected to dominate the market with revenue worth US$ 754.3 Mn by 2032.
“Increasing number of products in clinical pipeline is anticipated to drive the U.S. market for respiratory syncytial virus vaccines,” says a Persistence Market Research analyst.
Market Competition
Key manufacturers of respiratory syncytial virus (RSV) vaccines are focusing on inorganic growth by undergoing clinical trials on RSV vaccines for expansion of their market share and presence, along with strengthening their capabilities with broader offerings to meet growing market demand.
This approach helps in the development of cost-effective vaccines with higher efficacy and performance, which is expected to drive market growth during the forecast period (2022 to 2032).
- In February 2022, GlaxoSmithKline plc announced a voluntary pause in the trial enrolment and vaccination evaluating a potential RSV maternal vaccine candidate in pregnant women.
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the U.S. respiratory syncytial virus vaccines market in its latest study, presenting historical demand assessment of 2017 – 2021 and projections for 2022 – 2032.
The research study is based on the drug (Palivizumab, RSVpreF PF06928316, RSVPreF3 OA (GSK3844766A), mRNA-1345, mRNA-1345, Janssen RSV Vaccine, ResVax, Nirsevimab (MEDI8897), and MVA-BN RSV) and distribution channel (institutional sales, retail sales, and online sales), in the U.S.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/33045
About Us: Persistence Market Research
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com


