Human Papillomavirus Testing Market 2022
The Human Papillomavirus Testing Market is destined to reach US$ 1,100 Mn at a CAGR of 7.6% between 2025. Though disease testing isn’t looked upon as thrilling as therapeutics, the turbulence that it witnesses can’t be ignored. In other words, disease testing is now used for supporting drugs’ clinical development, foretell disease even prior to showing up of symptoms, and also identify the patients who could be responsive to the prescribed treatments. Companion diagnostics are being used by the medical practitioners for helping in selection of appropriate drugs for appropriate patients. Genomics are also brought into cancer care. With continuous monitoring, the disease testing market is bound to grow atrociously in the near future.
Get Free Sample Copy Of Human Papillomavirus Testing Market Report@ https://www.persistencemarketresearch.com/samples/11737
Various healthcare and government insurance providers are offering reimbursement for diagnosis of chronic disorders such as cervical cancer screening. For instance, Medicare – U.S. government-funded health insurance provider, provides reimbursement to people who are of age 65 or older and some younger population with disabilities.
Moreover, for cervical cancer diagnosis, such plans cover reimbursement for one pap test every three years or a combination of pap test & HPV testing every five years. Favorable government initiatives as such are expected to drive the demand for Human Papillomavirus (HPV) testing market in the years to come.
Company Profiles
- F. Hoffmann-La Roche Ltd.
- Agilent Technologies, Inc.
- Becton, Dickinson and Company
- Qiagen N.V.
- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Hologic Inc.
- Cepheid Inc.
- SEEGene Inc.
- Takara Bio Inc.
- DaAn Gene Co., Ltd. of Sun Yat-Sen University
- Promega Corporation
- Greiner Bio-One International GmbH (Greiner Holding AG)
- Enzo Biochem Inc.
- Norway Biotek Corp.
- DiagCor Bioscience Inc Ltd
- Hybribio Limited
- Zytovision GmbH
- Arbor Vita Corporation
- Medical & Biological Laboratories Co., Ltd
- Fujirebio Diagnostics, Inc.
- Others.
How About Step-By-Step Insights To Human Papillomavirus Testing Market? Look Through The “Methodology” Employed! https://www.persistencemarketresearch.com/methodology/11737
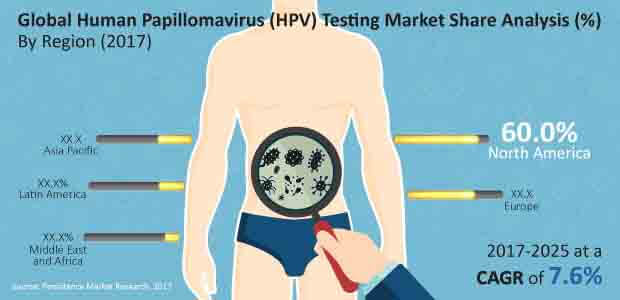
HPV testing is gradually becoming crucial for cervical cancer screening, according to recent report published by Persistence Market Research.
The report estimates that in 2017, the global market for Human Papillomavirus testing will reach an estimated value of US$ 629.4 Mn. Improving reimbursement scenario, coupled with high sensitivity of Human Papillomavirus testing in low resource setting, is expected to drive the market’s growth.
By the end of 2025, the global market for Human Papillomavirus testing will have soared at 7.6% CAGR, bringing in more than US$ 1,100 Mn in revenues.
Global HPV Testing Market – Key Challenges
A key limitation for Human Papillomavirus testing is that these tests are confined to women as men undergoing HPV testing have higher risks of getting infected with genital warts. In addition to this, patients are not willing to take Human Papillomavirus tests because of lack of diagnosis options.
Over-diagnosis of Human Papillomavirus testing can cause more complications and can even risk the life of patients. Meanwhile, the global revenues from Human Papillomavirus testing are being lowered due to availability of HPV vaccines.
Preference to HPV vaccines is directly impeding the revenues arising from Human Papillomavirus testing. High cost of HPV screening test, overburdened healthcare system, low awareness on HPV prevention, and imperfections in organized screening programs are some other challenges impacting the growth of global HPV testing market.
According to the report, North America’s Human Papillomavirus testing market will be valued at nearly US$ 640 Mn, dominating the global market revenues.
Purchase Our Human Papillomavirus Testing Market Report Now@ https://www.persistencemarketresearch.com/checkout/11737
The demand for Human Papillomavirus testing will also be significant in Europe, Latin America and Asia-Pacific, while Middle East & Africa’s Human Papillomavirus testing revenues will attribute to less than 4% of global market value.
The report also projects that over 60% of global HPV testing revenues will be accounted by DNA-based test products. While hospitals will be largest end-users of HPV testing globally, the end-use of Human Papillomavirus testing will gain traction in diagnostic centers as well.
And, the report also reveals that close to 90% of global Human Papillomavirus testing market value throughout the forecast period will be accounted by molecular diagnostic application of Human Papillomavirus testing.
About Us:
Persistence Market Research (PMR), as a 3rd-party research organization, does operate through an exclusive amalgamation of market research and data analytics for helping businesses ride high, irrespective of the turbulence faced on the account of financial/natural crunches.
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com


